Loading
Get Rapicide Log Sheet
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Rapicide Log Sheet online
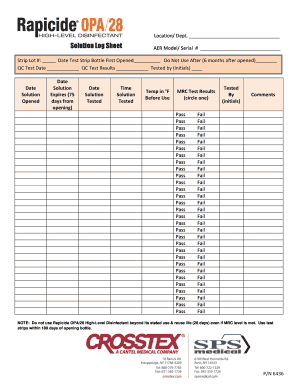
The Rapicide Log Sheet is essential for tracking the usage and testing of Rapicide solutions. This guide provides clear, step-by-step instructions to help users fill out the form accurately and efficiently online.
Follow the steps to complete the Rapicide Log Sheet correctly.
- Press the ‘Get Form’ button to obtain the form and access it in your preferred online editor.
- In the first field, enter the location or department associated with the use of the Rapicide solution.
- Fill in the AER model or serial number of the device being used in the designated field.
- For the Strip Lot number, provide the lot number associated with the test strip used, and ensure to record the date when the test strip bottle was first opened.
- Indicate the expiration date for the test strips, which is six months after opening, in the appropriate space.
- Complete the quality control (QC) test date and results fields by entering the date when the test was conducted and the outcome of the test.
- Initial the form to indicate who performed the testing in the 'Tested by' section.
- Continue filling the form by recording the date when the solution was opened, its expiration date (75 days from opening), date of testing, time of testing, and temperature in Fahrenheit before use.
- For MRC test results, circle the appropriate result, either 'Pass' or 'Fail', and ensure that initials of the individual who performed the test are noted.
- Utilize the comments section for any additional remarks, noting any observations or issues encountered during the testing process.
- Once all fields are accurately filled, save the changes to your document and utilize the options available to download, print, or share the Rapicide Log Sheet as needed.
Complete your Rapicide Log Sheet online today to ensure proper documentation and compliance.
Shelf life is one year unopened and 90 days once opened. The entire indicator test strip pad, except for the top 2 mm, must be completely red to PASS, indicating the concentration of the RAPICIDE Glutaraldehyde is above the MRC of 1.5%.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


