Loading
Get Acids And Bases Ws 4 Ph And Poh Answer Key
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Acids And Bases Ws 4 Ph And Poh Answer Key online
This guide provides clear and detailed instructions on how to complete the Acids And Bases Ws 4 Ph And Poh Answer Key online. Follow these steps to ensure accurate and efficient completion of the form.
Follow the steps to fill out the form effectively.
- Click the ‘Get Form’ button to obtain the Acids And Bases Ws 4 Ph And Poh Answer Key form and open it in your preferred online editor.
- In the first section, input your name in the designated field labeled 'Name'. This ensures your responses are attributed correctly.
- Next, enter the date in the section labeled 'Date' to document when the form is being filled out.
- Proceed to the 'Period' field, where you should input the relevant period for the assignment.
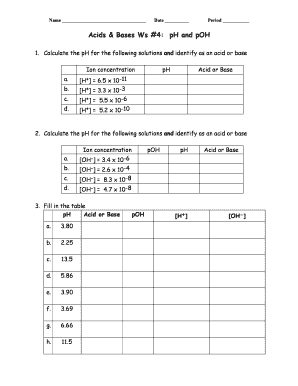
- For question 1, calculate the pH values for the provided hydrogen ion concentrations. You can identify whether each solution is an acid or a base by the pH value calculated.
- In question 2, repeat the process for calculating pH based on the given hydroxide ion concentrations, and identify the solutions as acids or bases.
- Question 3 involves filling in a table based on additional pH values and their classifications as acids or bases. Ensure all fields are accurately filled out as per the calculations.
- After completing all sections, you can save your changes. The form may allow you to download, print, or share it as needed.
Complete your documents online today for efficient and organized management.
Correct answer: Explanation: pH and pOH are the log concentrations of protons and hydroxide ions, respectively. The sum of pH and pOH is always 14. This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant for the ionization of water, which is equal to .
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


