Loading
Get Form Fda 3519 Fda National Registry Report - Fda
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the FORM FDA 3519 FDA National Registry Report online
Filling out the FORM FDA 3519 is essential for ensuring effective communication with the FDA regarding food safety programs. This comprehensive guide provides a clear step-by-step approach to assist you in completing the report accurately and efficiently.
Follow the steps to complete the FORM FDA 3519 online.
- Press the ‘Get Form’ button to obtain the form and open it in your selected editor.
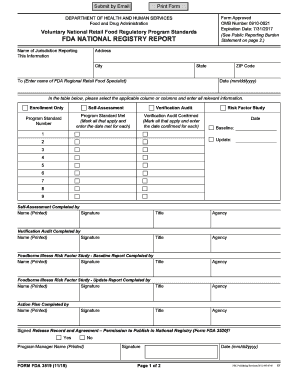
- In the reporting jurisdiction section, enter the name of the jurisdiction and its address. Then, select the name of the FDA Regional Retail Food Specialist responsible for this jurisdiction and provide the date of submission in the specified format (mm/dd/yyyy).
- Complete the table by selecting the applicable columns. For Enrollment Only, check the box if applicable. For Self-Assessment, indicate if the Program Standard(s) were met during the self-assessment along with the corresponding dates.
- Continue filling out the Verification Audit section by marking the relevant columns to indicate which Program Standard(s) have been confirmed, including the dates confirmed.
- For the Risk Factor Study section, check the corresponding box and enter dates for both the Baseline Survey and the most recent Risk Factor Study update if applicable.
- Identify and provide details for persons completing the various activities: Self-Assessment, Verification Audit, Foodborne Illness Risk Factor Study - Baseline Report, Foodborne Illness Risk Factor Study - Update Report, and Action Plan. Include their names, signatures, titles, and agencies.
- Indicate if you have submitted or will submit Form FDA 3520 by checking 'YES' or 'NO' in the Signed Release Record and Agreement section.
- Lastly, enter the name of the Program Manager, provide a signature (either electronically or by hand), and date the form when it is signed.
- After completing the form, you can save any changes, download it for your records, print it for submission, or share it with the necessary parties.
Start completing the FORM FDA 3519 online today to ensure your food safety programs are accurately reported.
Contact CDC about a Foodborne Illness or Outbreak: Please call CDC INFO at 1-800-CDC-INFO (1-800-232-4636).
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


