Loading
Get 13 Electron Configuration-s - Mrsrutschilling Mndhs
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the 13 Electron Configuration-S - Mrsrutschilling Mndhs online
The 13 Electron Configuration-S - Mrsrutschilling Mndhs is a valuable online tool designed to help learners understand the electron structures of atoms. This guide provides clear instructions on how to effectively complete the form to enhance your understanding of electron configurations.
Follow the steps to successfully fill out the form.
- Click the ‘Get Form’ button to access the 13 Electron Configuration-S - Mrsrutschilling Mndhs form and open it for editing. This will facilitate your ability to complete the necessary information accurately.
- Begin by reviewing the introductory section of the form, which outlines the purpose and importance of electron configurations in chemistry. Familiarize yourself with key terms mentioned to ensure a thorough understanding of the topic.
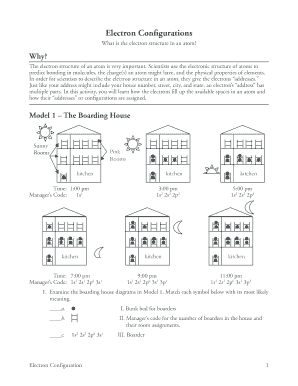
- Proceed to fill in the first section on the boarding house analogy provided in Model 1. You will need to examine and match each symbol with the correct meaning as indicated. Use the diagrams to assist you in making your selections.
- Answer the specific questions regarding the number of boarders present at designated times and provide explanations as outlined in the questions. This will require detailed observational analysis of the models presented.
- In the next section, utilize the orbital diagrams and electron configurations from Model 2. Carefully reproduce the underlining, circling, and boxing activities requested to effectively convey your understanding of the electron configuration.
- Follow the instructions related to the Pauli exclusion principle and Hund’s rule in the designated areas of the form. Be sure to circle the correct answers as these principles are vital to understanding electron configurations.
- Finally, review all your entries for accuracy. Upon completion, you can save your changes, download the form, print it, or share it as needed.
Start completing your 13 Electron Configuration-S - Mrsrutschilling Mndhs form online today!
ElementAtomic numberElectron configurationaluminum131s22s22p63s23p1silicon141s22s22p63s23p2phosphorus151s22s22p63s23p3sulfur161s22s22p63s23p414 more rows
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


