Loading
Get The Arrive Guidelines Checklist - Filesfigsharecom
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the The ARRIVE Guidelines Checklist - Filesfigsharecom online
The ARRIVE Guidelines Checklist is a critical tool for reporting in vivo animal research, ensuring transparency and reproducibility. This guide provides a comprehensive overview and step-by-step instructions on how to effectively complete the checklist online.
Follow the steps to fill out the ARRIVE Guidelines Checklist with ease.
- Press the ‘Get Form’ button to access the checklist and open it for editing.
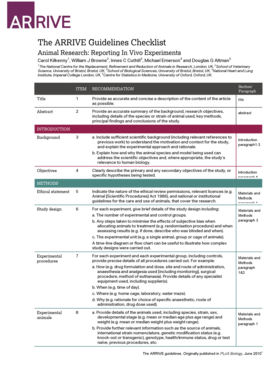
- Begin with the title section. Provide a precise and accurate title that reflects the content of your article.
- Move to the abstract field where you summarize the background, objectives, species or strain of animal used, key methods, findings, and conclusions.
- In the objectives section, clearly outline the primary and secondary objectives or any specific hypotheses being tested.
- Complete the ethical statement by indicating the nature of the ethical review permissions and any relevant national or institutional guidelines.
- In the study design section, briefly describe the design including the number of experimental and control groups, methods used to minimize bias, and the experimental unit.
- Detail all experimental procedures, specifying aspects such as drug formulation, administration routes, and any specialist equipment utilized.
- Provide comprehensive information about the experimental animals, including species, strain, sex, developmental stage, and genetic modification status.
- Share housing and husbandry details, including type of facility, husbandry conditions, and welfare considerations.
- Specify the total number of animals used and how that number was determined, along with the number of independent replications.
- Explain how animals were allocated to experimental groups, detailing randomisation or matching methodologies.
- Define the primary and secondary experimental outcomes that will be assessed.
- Provide details about the statistical methods used for each analysis and how data assumptions were evaluated.
- Report baseline data for each experimental group to characterize the health status of animals prior to treatment.
- State the number of animals included in each analysis clearly, providing absolute numbers.
- Report results with precision measures such as standard error or confidence interval.
- Detail significant adverse events in each experimental group and any protocol modifications made.
- Interpret results in the context of study objectives and current scientific literature, addressing limitations and implications.
- Discuss the generalizability of findings to other species or systems, especially any relevance to human biology.
- Finally, list all funding sources and any roles the funders had in the study.
- Once all fields are completed, save your changes. You can choose to download, print, or share the completed form.
Complete your documents online today to ensure clarity and compliance with the ARRIVE guidelines.
2.9. At present, FM Radio broadcasting in 88-108 MHz frequency band is permitted to All India Radio (AIR); private sector FM Radio broadcasters; and Community Radio Station (CRS) operators. AIR is the public service broadcaster under Prasar Bharti.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


