Loading
Get Ich Gcp Essential Document Checklist - Welcome To Urmc - Urmc Rochester
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the ICH GCP Essential Document Checklist - Welcome To URMC - Urmc Rochester online
The ICH GCP Essential Document Checklist is a crucial tool for managing essential documents in clinical trials. This guide provides step-by-step instructions to assist users in accurately completing the checklist online, ensuring adherence to regulatory standards.
Follow the steps to complete the checklist effectively.
- Click the ‘Get Form’ button to obtain the form and open it in the editor.
- Begin by entering the study number and selecting the study status. This information is essential for identifying the specific trial and its current state.
- Indicate the study type. Choose from clinical drug trial or non-drug subject research options. This categorization helps in organizing the documents relevant to each type.
- Fill out the necessary sections according to the study's phase. Include items such as the pre-activation requirements, active study requirements, and closing items, ensuring all relevant documents are accounted for.
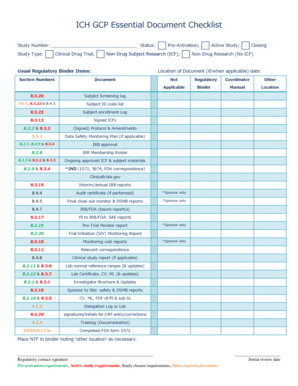
- For each item listed in the Usual Regulatory Binder, mark the applicable documents by checking the ‘Not Applicable’ box where necessary. This includes subject screening logs, signed informed consent forms, and data safety monitoring plans.
- Review the individual subject-related items, ensuring that source documents, signed/dated completed case report forms (CRFs), and relevant correspondence are documented properly.
- If applicable, complete the contract items section, which includes financial agreements and insurance statements. Ensure that any necessary supporting documents are also included.
- For pharmacy-specific items, if relevant, record the master randomization list and other pharmacy-specific documents as required by the study protocol.
- Before finalizing the form, ensure that the regulatory contact signature and initial review date are clearly indicated.
- Save your changes, and once completed, download, print, or share the checklist as needed for your records or submissions.
Complete your ICH GCP Essential Document Checklist online today for a smooth trial process.
ing to the ICH Guideline, Section 1.23, 'essential documents' are defined as: 'Documents which individually and collectively permit evaluation of the conduct of a study and the quality of the data produced'.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


