Loading
Get Mdsap Qms F00041001 Risk Management Process Flowchart - Fda
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to use or fill out the MDSAP QMS F00041001 Risk Management Process Flowchart - Fda online
Filling out the MDSAP QMS F00041001 Risk Management Process Flowchart - Fda is an essential step for organizations seeking to implement effective risk management processes. This guide provides clear, step-by-step instructions to help users navigate the form efficiently.
Follow the steps to complete the risk management process flowchart online.
- Click ‘Get Form’ button to obtain the form and open it in the editor.
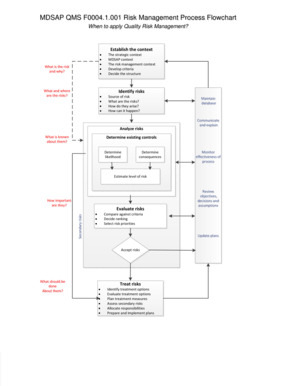
- Begin by establishing the context for the quality risk management. Clearly define the strategic and MDSAP context, as well as the specific risk management context that will guide your analysis.
- Develop criteria that will be used to evaluate risks. This involves deciding the structure of your criteria to ensure that all potential risks are considered adequately.
- Identify the risks by answering essential questions such as 'What are the risks?' and 'How do they arise?' Document sources of risks and their potential impacts.
- Analyze the identified risks by compiling known information about them. This will help in understanding the existing controls in place and the likelihood of risks occurring.
- Maintain a risk database to track identified risks and their statuses. Ensure regular updates to reflect current risk evaluations.
- Communicate and explain the risk management process to relevant stakeholders. Clear communication is vital for effective risk management.
- Determine the likelihood and consequences of each risk. This will assist in estimating the overall level of risk associated with each identified threat.
- Evaluate risks by comparing them against established criteria. Decide on ranking and prioritize risks based on their importance.
- Update plans as necessary based on the evaluation. It is vital to remain flexible and adjust plans according to the findings.
- Decide on which risks to accept and what actions should be taken about them. Review objectives, decisions, and assumptions as part of this process.
- Treat risks by identifying and evaluating treatment options. Plan the necessary treatment measures, assess any secondary risks, and allocate responsibilities to appropriate personnel.
- Prepare and implement the treatment plans. Ensure all involved parties are aware of their roles and responsibilities.
- Once completed, users can save their changes, download, print, or share the form as required.
Start filling out your MDSAP QMS F00041001 Risk Management Process Flowchart - Fda online today to enhance your risk management strategies.
Here's an overview of the steps you'll need to follow to write your statement of work: Create a brief introduction for your project. Define the purpose of your project. Define your project scope. Create a work breakdown structure to identify your project tasks, milestones and deliverables. Create a project schedule.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


