Loading
Get How Are Ions Formed
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the How Are Ions Formed online
Filling out the How Are Ions Formed form online provides a structured way to understand the formation and characteristics of ions. This guide will walk you through each step to ensure a smooth experience while providing clarity on the scientific concepts involved.
Follow the steps to complete the online form
- Press the ‘Get Form’ button to access the form and open it in the designated platform.
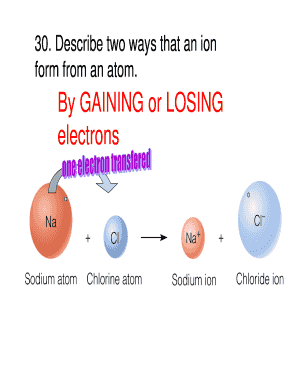
- Begin by addressing the first question, which typically involves describing two ways ions form from an atom. You may write about gaining or losing electrons.
- Proceed to the section where you need to name each ion based on the provided examples. Correctly identify each ion as an anion or cation, using the format shown in the document.
- Fill out the part that asks about the number of electrons in each atom and the respective group each belongs to. Reference the examples given for guidance.
- In the next part, answer how many electrons each atom must lose to achieve a noble gas electron configuration. Make sure to provide the correct number for each element listed.
- Explain why nonmetals tend to form anions, focusing on their tendency to fill their outer valence shell.
- Continue to the section on how many electrons each atom must gain to achieve stability, ensuring precise answers for each atom.
- Identify the kinds of ions that form each ionic compound as listed in the form, using the correct notation for each ion.
- Complete any additional questions regarding the formation of ionic compounds and properties such as conductivity.
- Once all sections are complete, review your entries for accuracy. After confirming everything is correct, you may save changes, download, print, or share the completed form as needed.
Start filling out the form online today to enhance your understanding of ion formation.
An ion is formed by the loss or gain of electrons by an atom, so it contains an unequal number of electrons and protons. Example: Sodium ion Na+, magnesium ion Mg2+, chloride ion Cl , and oxide ion O2 . There are two types of ions : cations.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


