Loading
Get Form Fda-2892 - Users Wpi
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Form FDA-2892 - Users Wpi online
Filling out the Form FDA-2892 is an essential step for registering your devices with the FDA. This guide provides a comprehensive overview and step-by-step instructions designed to assist users in completing the form accurately and efficiently.
Follow the steps to successfully complete the Form FDA-2892.
- Press the ‘Get Form’ button to access the form and open it for editing.
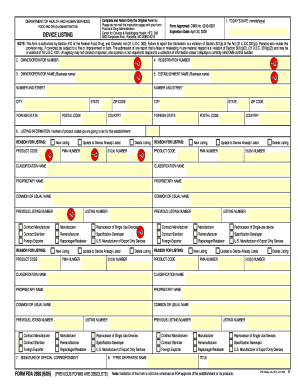
- Item 1: Enter today’s date in the format mm/dd/yyyy. This indicates when the form is completed.
- Item 2: Fill in the Owner/Operator Number if you have previously received an identification number from the FDA. Leave blank if not applicable.
- Item 3: Provide the Owner/Operator Name, which is the business name of the entity registering the establishment. Include the corresponding address following the General Address instructions.
- Item 4: Enter your Registration Number if assigned. If not, leave this field blank; the FDA will assign a number upon processing.
- Item 5: Complete the Establishment Name and Address section with the legal name and address of the establishment conducting regulated activities.
- Item 6: Indicate the number of products you are listing. For each product, provide necessary details like newer listing status, product codes, 510(k) or PMA numbers, and classification names.
- Item 7: Include the Signature of the Official Correspondent to validate the submission.
- Item 8: Print or type the name and title of the official correspondent.
- Review all completed fields for accuracy. Once finalized, you can save any changes made to the form, print a copy, or share as necessary.
Begin your journey in device registration online by completing the Form FDA-2892 now.
To align with PDUFA VII , BsUFA III, and GDUFA III requirements as well as make other improvements to data quality and ease of use, FDA has revised form 356h: Application to Market a New or Abbreviated New Drug or Biologic for Human Use and form 1571: Investigational New Drug Application (IND).
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


