Loading
Get Biosafety Application Approval Form - James Cook University - Jcu Edu
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Biosafety Application Approval Form - James Cook University - Jcu Edu online
This guide provides step-by-step instructions for users to effectively complete the Biosafety Application Approval Form online at James Cook University. By following these instructions, users will ensure that their application meets all necessary biosafety requirements.
Follow the steps to complete the biosafety application approval form online.
- Click ‘Get Form’ button to access the Biosafety Application Approval Form and open it for completion.
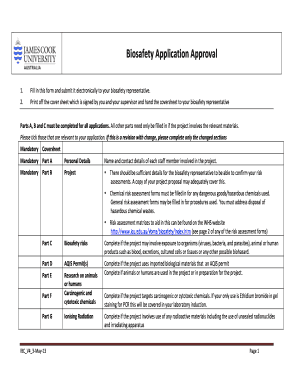
- Fill in the mandatory cover sheet with your details, including your name and contact information. Make sure to have the required signatures from both yourself and your supervisor.
- In Part A, provide personal details of all staff members involved in the project, including contact information and details of training received pertinent to biosafety.
- In Part B, describe the project title, commencement date, and duration. Include the aims and anticipated outcomes while ensuring all relevant information about chemicals and biosafety risks is documented.
- Complete Part C regarding biosafety risks if the project may involve exposure to hazardous organisms or biohazards.
- Part D addresses any AQIS permits required for imported biological materials. Be sure to attach copies of these permits.
- In Part E, answer questions regarding the use of animals and humans in your research and provide necessary ethics approvals.
- Complete Part F if the project involves carcinogenic or cytotoxic chemicals. Detail your methodology, storage, and classification of substances.
- For Part G, provide information about any ionising radiation used in the project, including details on handling and supervision.
- Finally, review all sections for accuracy, then save your changes, and electronically submit the completed form to your biosafety representative. You may also print or download the completed form for your records.
Ensure your biosafety application is complete by following these guidelines and submit your documents online today.
The Institutional Biosafety Committee (IBC) reviews: Proposals for laboratory and clinical studies involving the use of Genetically Modified Organisms (GMOs) to ensure the research meets the requirements of the Gene Technology Act 2000 and the Gene Technology Regulations 2001.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


