Loading
Get Richmond Environmental Coc Form - Microbac Laboratories, Inc.
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Richmond Environmental COC Form - Microbac Laboratories, Inc. online
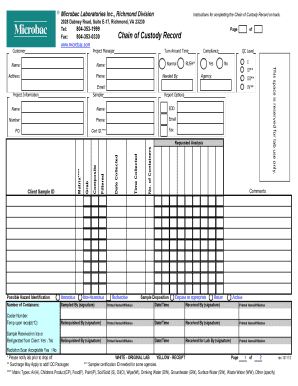
The Richmond Environmental COC Form is essential for documenting the chain of custody for environmental samples submitted to Microbac Laboratories. This guide provides clear, step-by-step instructions on how to accurately complete the form online for efficient processing.
Follow the steps to complete the form online effectively.
- Click ‘Get Form’ button to obtain the Richmond Environmental COC Form and open it in your preferred form editor.
- Begin filling out the customer information section at the top of the form. Enter the project manager's name, address, and phone number to ensure proper communication.
- Indicate the turnaround time needed by selecting either 'Normal' or 'RUSH' as applicable. If selecting 'RUSH', notify the lab prior to sample drop-off.
- Select the appropriate quality control level from the options provided, ensuring you understand the implications of each QC level on your results.
- Fill in the project information section, specifying the project name and location. This is important for report generation.
- Input your Purchase Order (PO) number for billing identification and tracking of services.
- Identify whether the samples are for compliance monitoring by answering 'Yes' or 'No', and indicate the relevant program if applicable.
- Choose the desired report type according to your requirements: Level I, Level II, Level III, or Level IV, as these indicate varying degrees of analysis detail.
- Provide the details of the sampler, including their name, contact number, and email address to ensure accountability in the sampling process.
- Specify how you would like to receive the final report by selecting your preferred means for report delivery.
- Provide a unique Client Sample ID to identify your samples on the report for ease of reference.
- Indicate the matrix type of your samples and whether they are collected as grab or composite samples, along with any filtering that occurred before delivery.
- Document the date and time the samples were collected to maintain an accurate chain of custody.
- List the number of containers used and any preservatives applied to the samples, adhering to the provided key for proper identification.
- Clearly state the requested analyses for the samples, specifying analytes and methods when possible for clarity.
- Identify any possible hazards associated with the samples and determine your desired sample disposition—a crucial aspect for safe handling.
- Use the comments section to communicate any additional information that may assist the lab in processing your samples.
- Sign the 'Relinquished By' field and record the date and time when the samples are passed into custody for tracking purposes.
- Finally, check the total number of pages included to ensure that all documentation is accounted for before submission.
- Once all sections are complete, save your changes, and remember to download, print, or share the completed form as needed.
Complete your documentation online today to ensure a smooth sample submission process.
Microbac provides quality and safety testing throughout the product life cycle for large and small molecule drugs. We have decades of experience working with pharmaceutical and biopharmaceutical products in compliance with GLP and cGMP regulation.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


