Get Estimated Dates Of Possible First Time Generics Form
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Estimated Dates Of Possible First Time Generics Form online
Filling out the Estimated Dates Of Possible First Time Generics Form online is a straightforward process that helps ensure accurate documentation of generic drug availability. This guide will provide you with step-by-step instructions to efficiently complete the form and submit it with confidence.
Follow the steps to complete the form effectively.
- Click ‘Get Form’ button to obtain the form and open it in the editor.
- Once the form is open, begin by reviewing the initial instructions provided, which will guide you through the various sections.
- Complete the first section that requests basic information, including the drug product name and any associated approvals or applications.
- Proceed to the section detailing the estimated dates for the first time generics, ensuring you provide accurate and realistic timelines based on your knowledge and resources.
- Next, review the information for accuracy before proceeding to any additional comments or supporting information section if applicable.
- Finally, save your changes and choose to download, print, or share the completed form as needed.
Start your document filing online today!
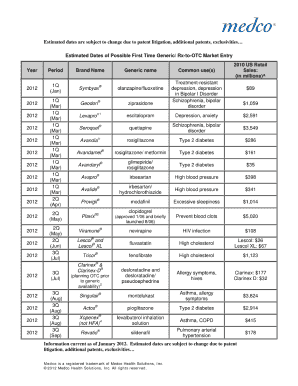
The timeline for FDA approval of new drugs can vary but generally takes several years. After initial research and development, a drug must undergo preclinical testing, followed by several phases of clinical trials. Once these steps are completed, the manufacturer submits a New Drug Application (NDA) for FDA reviews. Keeping track of the Estimated Dates Of Possible First Time Generics Form can help you stay informed about potential market entry for generics.
Industry-leading security and compliance
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


