Loading
Get Source Data Verification Checklist
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Source Data Verification Checklist online
The Source Data Verification Checklist is an essential tool for ensuring the accuracy and completeness of clinical study data. This guide provides step-by-step instructions to assist users in filling out the checklist online effectively.
Follow the steps to complete the checklist with ease.
- Press the ‘Get Form’ button to access the checklist and open it in the online editor.
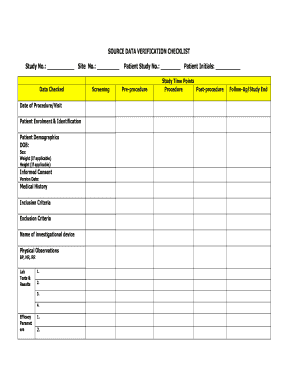
- Begin by entering the study number and site number at the top of the form. These identifiers are crucial for tracking your data accurately.
- In the 'Data Checked' section, input the date of the procedure or visit. This helps establish a timeline for the data being verified.
- Fill in the 'Patient Enrolment & Identification' section with the relevant patient demographics, such as date of birth, sex, weight (if applicable), and height (if applicable).
- In the 'Informed Consent' section, enter the version date of the consent form to ensure compliance with ethical standards.
- Provide the medical history along with the inclusion and exclusion criteria, which are vital for assessing the eligibility of the study subjects.
- Document the name of the investigational device and any physical observations including blood pressure, heart rate, and respiratory rate.
- In the 'Lab Tests & Results' section, list all relevant tests conducted and their results, numbering them for clarity.
- Complete the efficacy parameters by providing specific metrics related to the study’s outcomes.
- Fill in the screening information including patient study number and initials to uniquely identify each participant.
- Document all data associated with the procedure, including pre-procedure, study time points, and post-procedure observations.
- Record any unscheduled visits, study end/discharge details, complications/adverse events, and serious adverse events, as well as concomitant medications.
- Add any comments or notes that provide additional context for the data being verified.
- Make sure no fields are left blank; if a field is not applicable, annotate it with 'n/a'.
- Sign the checklist at the bottom of the form, noting the date of signature. This signifies that the monitor has completed the verification process.
- Finally, save your changes, and you can download, print, or share the completed form as necessary.
Complete your Source Data Verification Checklist online now for efficient data management.
Data verification is done through a process of meticulous checks against standard criteria and benchmarks. It often requires a combination of manual reviews and automated systems to ensure accuracy. By implementing a Source Data Verification Checklist, you can streamline this process, guaranteeing that your data is sound and trustworthy.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


