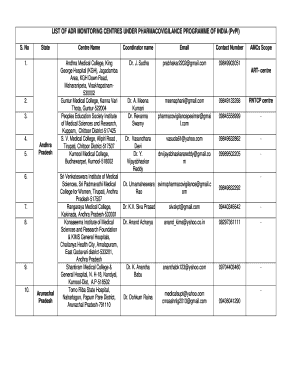
Get List Of Adr Monitoring Centres Under Pharmacovigilance Programme Of India (pvpi)
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the LIST OF ADR MONITORING CENTRES UNDER PHARMACOVIGILANCE PROGRAMME OF INDIA (PvPI) online
This guide provides a comprehensive and user-friendly approach to filling out the LIST OF ADR MONITORING CENTRES under the Pharmacovigilance Programme of India (PvPI) online. Each step is designed to ensure clarity and support as you complete the form.
Follow the steps to fill out the form accurately.
- Click ‘Get Form’ button to access the form and open it in your document editor.
- Begin by entering the serial number (S. No) of the monitoring centre. This helps in maintaining an organized record.
- In the 'State' field, specify the name of the state where the monitoring centre is located.
- In the 'Centre Name' section, enter the official name of the monitoring centre as recognized in official records.
- Fill in the 'Coordinator Name' field with the full name of the individual responsible for the centre. This is essential for point of contact.
- Provide the email address of the coordinator in the 'Email' field. Ensure that this is a professional address linked to the monitoring centre.
- In the 'Contact Number' field, include a reachable phone number for the coordinator. This is important for communication regarding drug safety issues.
- If applicable, select and indicate the scope of the AMC in the 'AMCs Scope' section. You may include categories such as ART-centre or RNTCP centre as relevant.
- Review all information for accuracy. Double-check that all fields have been filled out correctly to avoid delays in processing.
- Once all information is complete, save changes to the document. You may also choose to download, print, or share the form as needed.
Complete and submit your documents online to ensure compliance with the Pharmacovigilance Programme.
There are various methods for reporting Adverse Drug Reactions (ADRs), including online forms, physical submissions at monitoring centers, and through healthcare professionals. Utilizing digital platforms makes the process smoother and quicker. For specific details, refer to the resources available in the LIST OF ADR MONITORING CENTRES UNDER PHARMACOVIGILANCE PROGRAMME OF INDIA (PvPI). Each method aims to facilitate effective communication regarding drug safety.
Industry-leading security and compliance
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


