Get Consort 2010 Checklist Of Information To Include When Reporting A Randomized Trial A 2020-2025
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the CONSORT 2010 Checklist of Information to Include When Reporting a Randomized Trial A online
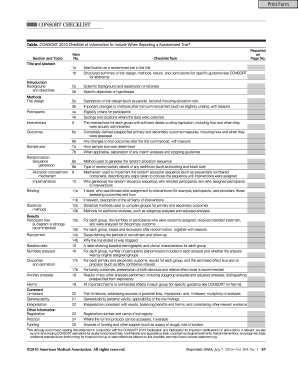
The CONSORT 2010 Checklist is an essential tool for researchers reporting randomized trials, ensuring all critical information is communicated effectively. This guide provides step-by-step instructions to help users fill out the checklist online, promoting clarity and thoroughness in trial reporting.
Follow the steps to complete the CONSORT 2010 checklist accurately.
- Press the ‘Get Form’ button to access the form and open it in your preferred editing interface.
- Begin with the title and abstract section. Ensure that your trial is identified as randomized in the title. Use the structured format to summarize the trial's design, methods, results, and conclusions.
- In the scientific background section, provide the rationale for your study and specify the objectives or hypotheses of the trial.
- Detail the interventions, including descriptions for each group. Mention sufficient information to enable replication, such as how and when the interventions were administered.
- Outline the outcomes, ensuring all primary and secondary measures are clearly defined and describe any changes to these measures after commencement.
- Indicate the sample size determination, documenting how it was established and any interim analyses that may be relevant.
- Complete the randomization section, describing the method and mechanism used for allocation concealment, as well as the blinding approach for participants and assessors, if applicable.
- In the results section, include participant flow, recruitment details, baseline data, and the analysis of outcomes. Provide a diagram if possible to enhance understanding.
- Summarize the trial limitations and discuss the generalizability of your findings, ensuring interpretations reflect the balance of benefits and harms.
- Finalize by including important information such as the trial registration number, access to the full protocol, and details about funding sources.
- Once all sections are completed, ensure your changes are saved, then proceed to download, print, or share the form as required.
Complete the CONSORT 2010 checklist online to enhance the clarity and reliability of your randomized trial reporting.
Calculating the fragility score involves assessing the number of patients whose outcomes would need to change to reverse the conclusions of a trial. Typically, it demonstrates how resilient an outcome is in the face of alternative scenarios. This score can be beneficial for clinicians looking to gauge the robustness of trial results.
Industry-leading security and compliance
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


