Loading
Get Subatomic Particles For Atoms, Ions And Isotopes - Mr. Birrell 2020-2025
How it works
-
Open form follow the instructions
-
Easily sign the form with your finger
-
Send filled & signed form or save
How to fill out the Subatomic Particles For Atoms, Ions And Isotopes - Mr. Birrell online
This guide provides users with detailed instructions for completing the Subatomic Particles For Atoms, Ions And Isotopes - Mr. Birrell online. Whether you are familiar with the subject or new to it, this resource will help you navigate through each section effectively.
Follow the steps to complete the form accurately.
- Click ‘Get Form’ button to obtain the form and open it in your preferred editing tool.
- Begin by entering the name at the top of the form. This identifies the individual who is completing the worksheet.
- Next, record the date in the designated field. This information marks when the worksheet was filled out.
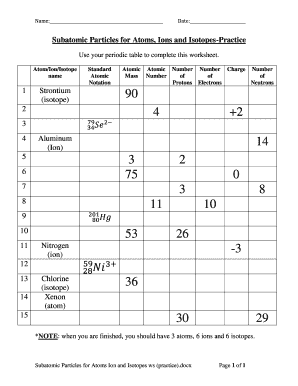
- Proceed to fill in the 'Atom/Ion/Isotope name' field. For each section, you will need to input the specific type of subatomic particle (i.e., Strontium, Aluminum, Nitrogen, Chlorine, Xenon) you are working with.
- In the following columns, fill out the standard atomic notation, including the atomic mass number, atomic number, charge, number of protons, electrons, and neutrons. Use the periodic table as a reference to find accurate values.
- Ensure that for each type of particle—atom, ion, and isotope— you correctly complete the requirements: 3 atoms, 6 ions, and 6 isotopes as indicated in the guidelines.
- Once all fields are completed, review your inputs to ensure accuracy and completeness.
- Finally, save your changes, and choose to download, print, or share the completed form as needed.
Start filling out your Subatomic Particles For Atoms, Ions And Isotopes form online today.
Labeling an atom involves denoting the number of protons, neutrons, and electrons it contains. You can represent this information using notation that combines the atomic symbol with the atomic number and mass number. Mr. Birrell’s guides provide clear frameworks for labeling atoms effectively and understanding the use of subatomic particles for atoms, ions, and isotopes.
Industry-leading security and compliance
US Legal Forms protects your data by complying with industry-specific security standards.
-
In businnes since 199725+ years providing professional legal documents.
-
Accredited businessGuarantees that a business meets BBB accreditation standards in the US and Canada.
-
Secured by BraintreeValidated Level 1 PCI DSS compliant payment gateway that accepts most major credit and debit card brands from across the globe.


